
Hans van der Jagt, in Encyclopedia of Analytical Science (Third Edition), 2019 Formation of ionic bondĪn ionic bond can be formed after two or more atoms loss or gain electrons to form an ion. The chloride ion has a − 1 charge because there are 17 protons in the nucleus, but there are 18 electrons around the nucleus of the ion. The chlorine atom, which has a high electronegativity, gains an electron and is converted into a chloride ion that has the same electron configuration as argon (1s 2 2s 22p 6 3s 23p 6). It has a + 1 charge, because there are 11 protons in the nucleus, but only 10 electrons around the nucleus of the ion. The resulting sodium ion has the same electron configuration as neon (1s 2 2s 22p 6). In forming an ionic bond, the sodium atom, which is electropositive, loses its valence electron to chlorine.

A chlorine atom, which has 17 protons and 17 electrons, has seven valence electrons in its third shell, represented as 3s 23p 5. A sodium atom, which has 11 protons and 11 electrons, has a single valence electron in its 3s subshell. Sodium chloride is an example of an ionic solid. When the solid dissolves, the ions dissociate and can diffuse freely in solution. Ionic substances exist as crystalline solids.

Electron transfer produces negative ions called anions and positive ions called cations.
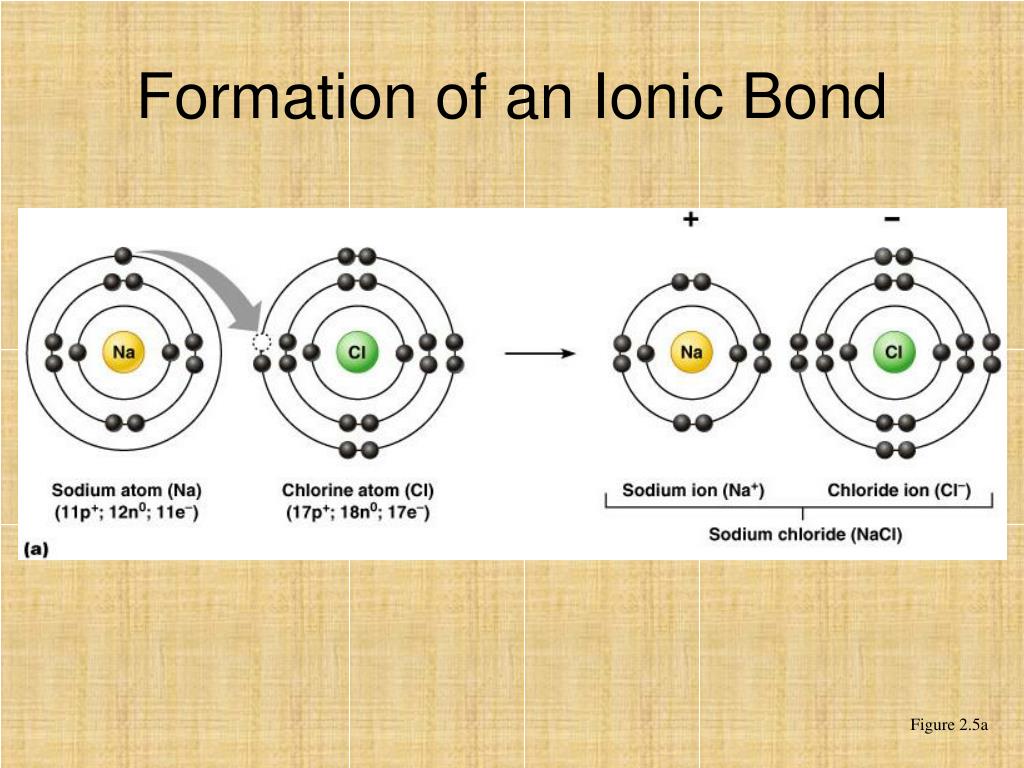
Ionic bonds are formed between two or more atoms by the transfer of one or more electrons between atoms. David Rawn, in Organic Chemistry (Second Edition), 2018 Ionic Bonds


 0 kommentar(er)
0 kommentar(er)
